History Of Batteries: A Timeline
1786: Frog Legs And Electricity
Luigi Galvani, an Italian physicist, found an indication that made ready to the possibility of the battery. Galvani was dismembering a frog appended to a metal snare with an iron surgical blade, and as he contacted the frog's leg, the leg jerked. The physicist trusted this was because of "creature power" wherein the vitality that started the development originated from the leg itself. This was significantly restricted by Alessandro Volta, who trusted that the wonder was caused by the two divergent metals and a sticky conduit. Volta checked this idea through a test, which he distributed in 1791.
1800: The Birth Of The Voltaic Pile
Volta took his examination further by making the main wet cell battery. Assembling layers of copper and zinc separated by layers of cardboard or material absorbed brackish water, Volta thought of what is currently known as the voltaic heap. The Voltaic Pile is the main genuine battery, creating a steady and predictable current. Be that as it may, in spite of being fit for conveying steady flows, the Voltaic Pile can't create power for quite a while. Volta's batteries just offer a short battery life, which is a hour's value at most extreme. One of its blemishes includes electrolyte spills which cause short circuits. Another issue is the development of hydrogen rises on the copper, expanding the inner opposition of the battery.
1836: The Daniell Cell Battery
English scientific expert, John Frederic Daniell prepared to conquering the Voltaic Pile's limitation by designing the Daniell Cell. Hydrogen bubbles were killed by utilizing a second electrolyte arrangement created by the primary conductor. The Daniell Cell made utilization of copper sulfate inundated in an unglazed ceramic vessel loaded up with a zinc anode and sulfuric corrosive. Since it was made out of permeable material, the pottery vessel enabled particles to go through yet kept the arrangements from blending. The Daniell Cell was additionally the primary battery to join mercury, used to diminish consumption. This battery type created 1.1 volts and was at first used to control specialized gadgets.
1838: The Porous Pot Cell
A Liverpool-based instrument producer, John Dancer, utilized the plan of the Daniell Cell. This battery was made out of a focal zinc anode drenched into a ceramic vessel containing an answer of zinc sulfate. The permeable ceramic pot is inundated in an answer of copper sulfate contained inside a copper can. The copper can goes about as the cell's cathode. Particles go through the permeable obstruction however the arrangements are kept from combining.
1838: The Fuel Cell
William Robert Grove developed the first fuel cell, which produced electrical by combining hydrogen and oxygen.

1859: The Arrival Of Lead Acid Batteries
All batteries recently developed were essential cells, thus they forever depleted after the entirety of their concoction responses were spent. Gaston Planté tackled this issue by making the primary battery that could be energized: the Lead-Acid Battery. By passing a charging and releasing current in the cell, the battery can supply vitality for a more drawn out time. A researcher named Camille Alphonse Faure upgraded the lead-corrosive battery. Faure structured a cell comprising of a lead network cross section in which the lead oxide glue was squeezed. Layers of these plate mixes were stacked for more prominent execution. The primary model for a lead-corrosive battery was made out of two lead sheets partitioned by elastic strips framing a winding. Lead-corrosive batteries were first used to control lights for train carriages.
1866: The Leclanché Cell, A Carbon-Zinc Battery
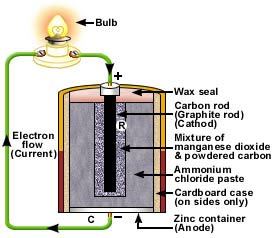
French researcher, Georges Leclanché concocted a battery made out of a zinc anode with a manganese dioxide cathode wrapped inside a permeable material. The cell made utilization of an ammonium chloride arrangement as the electrolyte. With carbon blended into the manganese dioxide cathode, this battery introduced quicker retention and longer time span of usability. Leclanché enhanced this battery by substituting the fluid electrolyte into a pastier form, which brought about the making of the main dry cell battery. It could be utilized in various introductions and transported without spilling.
1886: Carl Gassner’s Version Of The Leclanché Cell
Another adaptation of dry cell was created via Carl Gassner, who acquired a German patent on a variation of the Leclanché battery. Gassner made utilization of Plaster of Paris to make the ammonium chloride glue, blended with a little measure of zinc chloride with the end goal to draw out the battery's timeframe of realistic usability. Accordingly, the battery offered a more strong plan and gave 1.5 volts in full utilize. Gassner acquired a US Patent for this battery in 1887. Gassner's thought made ready for the principal mass-driven battery, fueling versatile electrical gadgets.
1899: The Nickel-Cadmium Battery
Waldermar Jungner, a researcher hailing from Sweden, has developed the principal nickel-cadmium battery (NiCD). This is a battery-powered battery containing nickel and cadmium terminals absorbed a potassium hydroxide arrangement. It is the main battery to make utilization of a soluble electrolyte, which thusly gives it the capacity to deliver preferred vitality thickness over the lead-corrosive battery.
1903: The Edison Battery
A popular American researcher, Thomas Edison, grabbed the nickel-press cell Jungner structured and made another licensed form of it. Edison made utilization of a soluble cell with iron as the anode and nickel oxide as the cathode. He additionally made utilization of potassium chloride as conductor. The Edison battery was at first gone for vehicles. In any case, it found more prominent use in the mechanical and railroad showcase, being solid enough to endure cheated and uncharged periods.
1954: The Solar Cells
Gerald Pearson, Calvin Fuller, and Daryl Chapin invented the first solar battery. A solar battery converts the sun's energy into electricity. The inventors created an array of several strips of silicon (each about the size of a razor blade), placed them in sunlight, captured the free electrons and turned them into electrical current. Bell Laboratories in New York announced the prototype manufacture of a new solar battery. Bell had funded the research. The first public service trial of the Bell Solar Battery began with a telephone carrier system (Americus, Georgia) on October 4, 1955.

1955: The Arrival Of Alkaline Batteries
Zinc-carbon batteries were the essential wellspring of vitality until the late 1950s. However, this battery type offers low time span of usability and can without much of a stretch be released. A specialist named Lewis Urry was appointed to discover an answer in expanding the life of zinc-carbon batteries by the Eveready Battery Company. Urry found that creation utilization of soluble in batteries offers more preferred standpoint, providing more noteworthy vitality at higher flows contrasted with the zinc-carbon batteries.
1992: Lithium And Lithium-Ion Batteries
Gilbert Newton Lewis began with the experimentation on lithium batteries however it was not until the last part of the century that the primary lithium batteries turned out to be financially accessible. Three essential advancements were indispensable to the formation of these batteries: the disclosure of the LiCoO2 cathode by John Goodenough (1980), the revelation of the graphite anode by Rachid Yazami (1982) and the battery-powered lithium battery model delivered by Asahi Chemical, Japan. Sony popularized the lithium particle battery in 1991.












